Efficient requirements management, standard-compliant documentation for regulated industries, complete traceability and test coverage over any number of releases: With the MedPack as an extension for Polarion, you save configuration effort and have access to an established solution. Of course, the extension can also be used outside of medical technology.
Features

Control
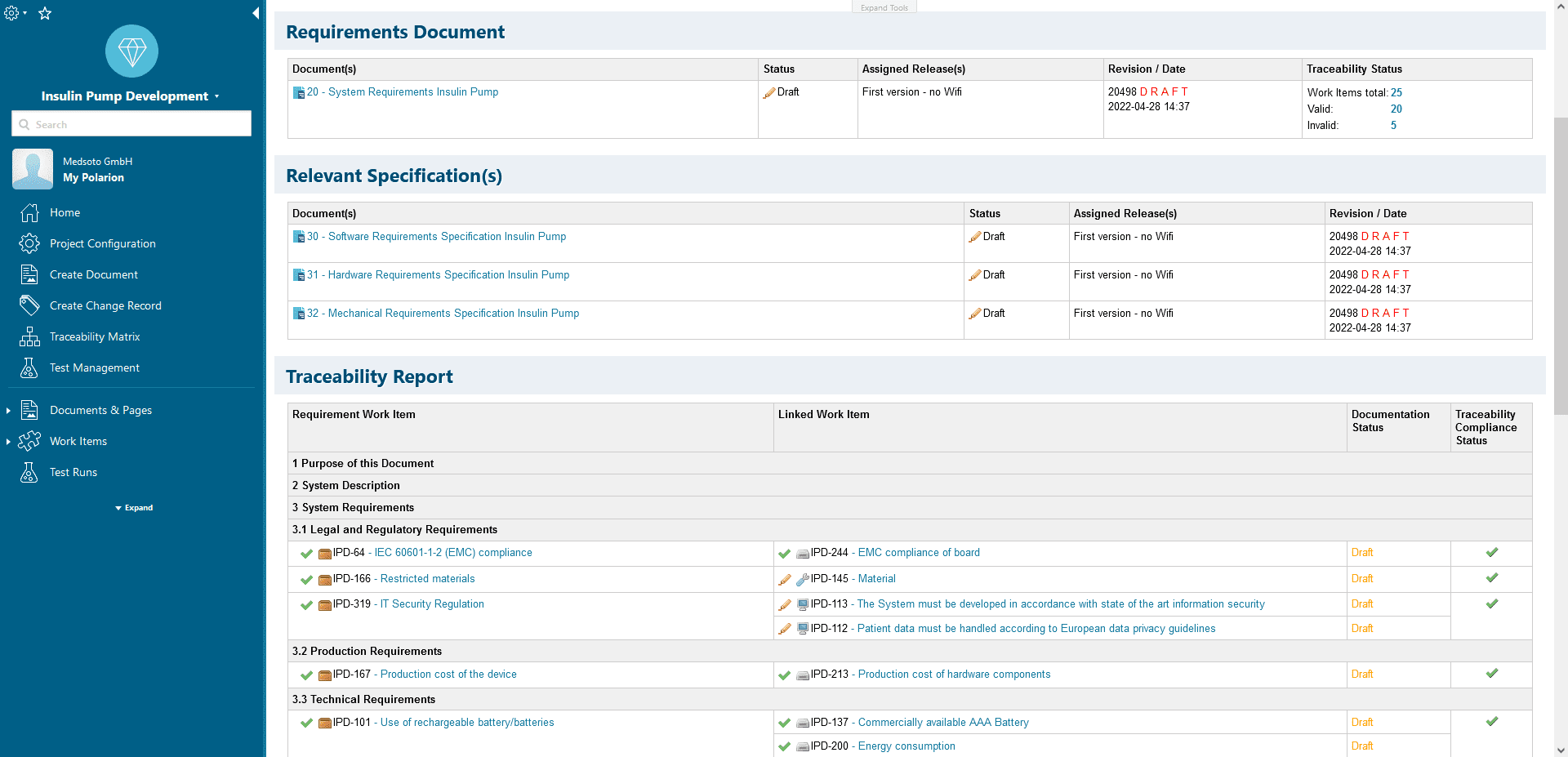
Quick overview of the completeness of your documentation and its conformity with IEC 62304

Documentation
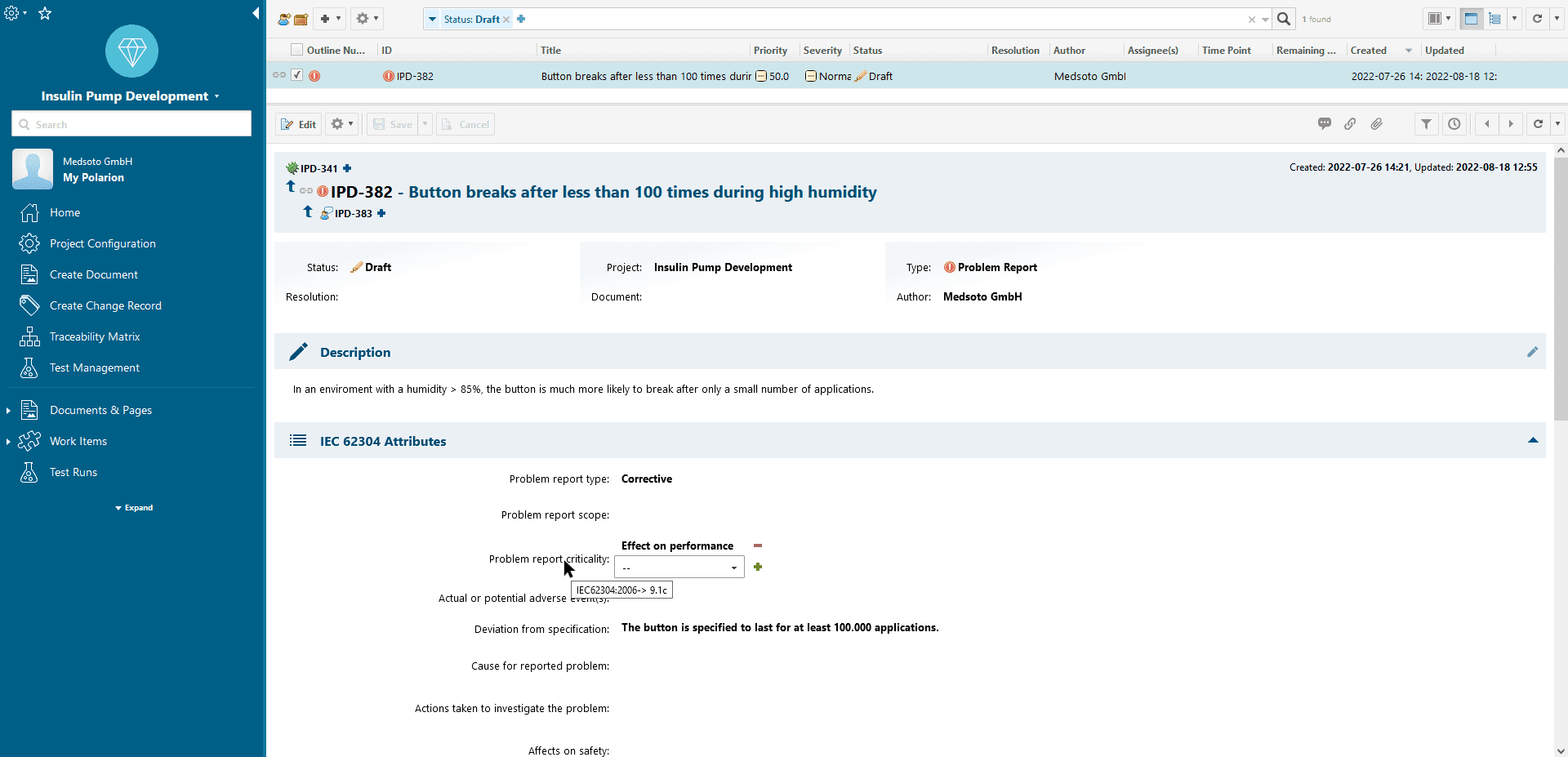
Easy documentation and linking of the activities required by IEC 62304

Efficient Product Development
The MedPack accompanies you through the complete software life cycle
Traceability Reports
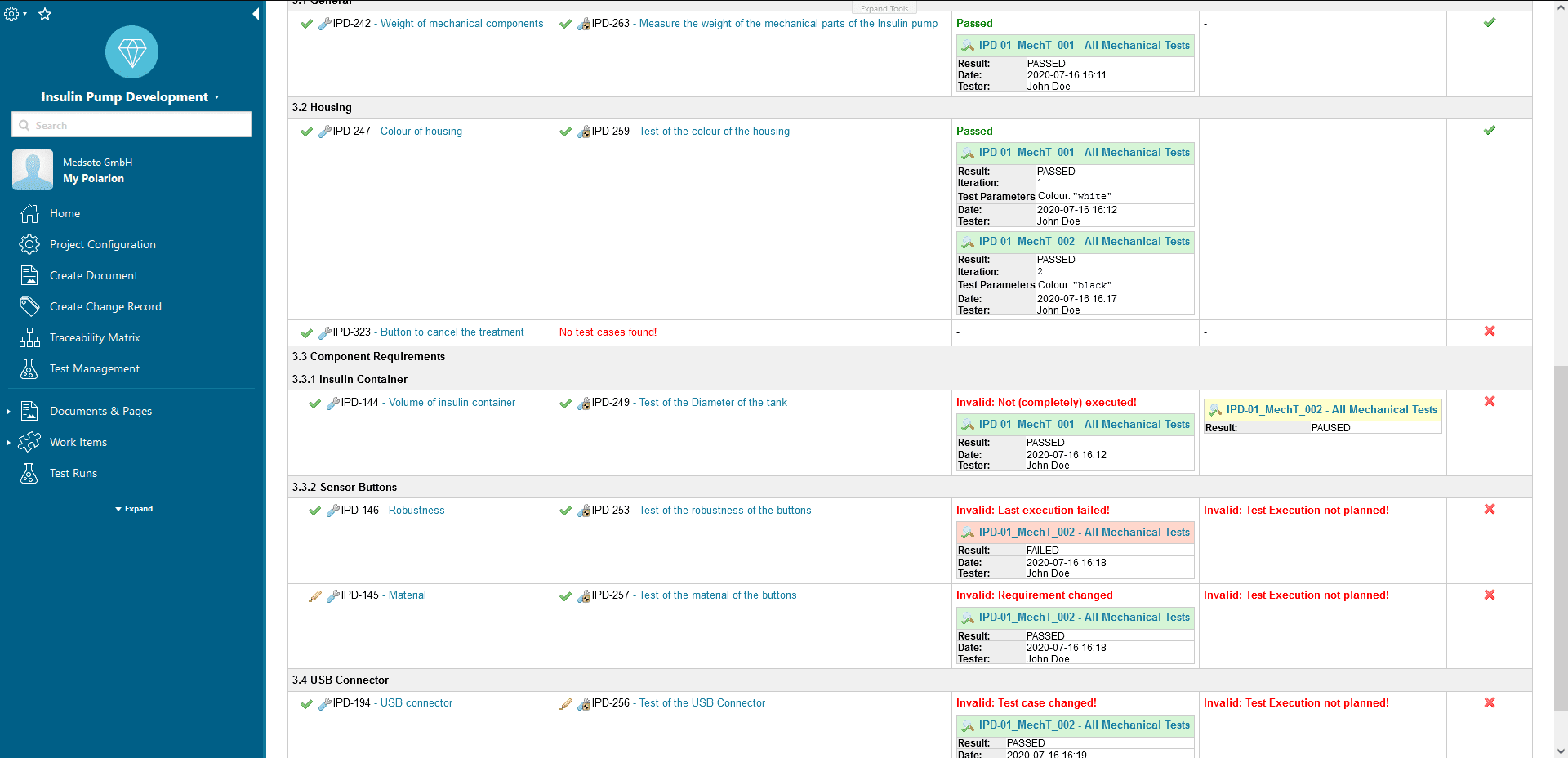
A structured data model makes it very easy to create the regulatory traceability reports, e.g. between requirements and tests.
Templates
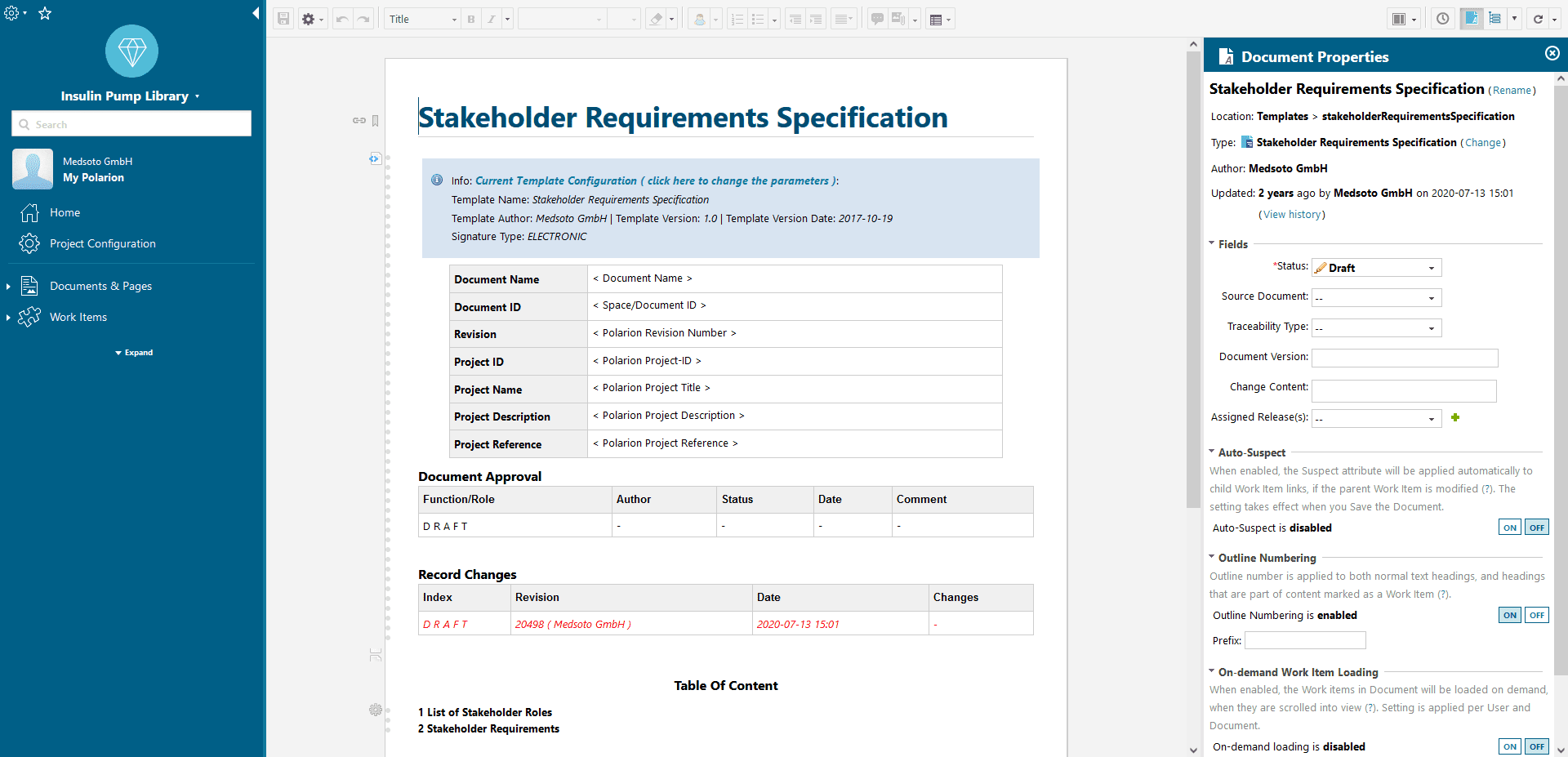
Templates for standard-compliant software and hardware development in medical technology. Simple documentation and linking of the activities required by IEC 62304.
Document Library
With the document library you manage your templates for your development documentation. It enables e.g. document control according to ISO 13485.
Consulting & contact
Sie wollen mehr wissen zu Traceability und normenkonformer Entwicklung?
Further products
Polarion ALM
With Polarion, you digitise and optimise your development processes in all phases of the product life cycle. Define, develop, test and manage complex systems. Transfer documents into structured digital documentation.
RiskPack
Document your risk management fully digitally and compliant with ISO 14971. RiskPack enables you to have an audit-proof risk management file, fully integrated in Polarion.